Rebuild. Rejuvenate. Regrow.
Regenerative
Plasma Concentrate
Advanced Bioactive Plasma
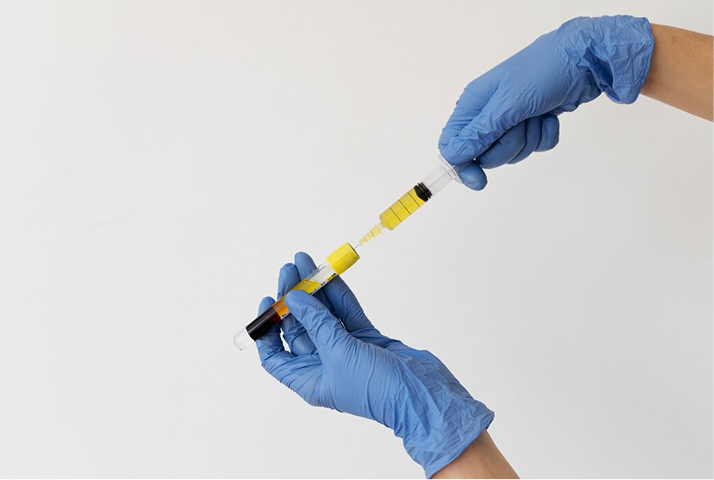
What is Regenerative Plasma Concentrate (RPC)?
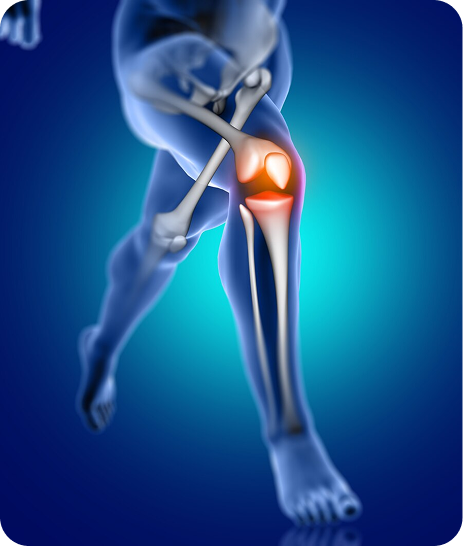
What is Regenerative Plasma Concentrate (RPC) Used to Treat?
- Partial Tendon / Ligament Tears
- Joint Repair
- Cartilage Disorders
- Osteoarthritis / Osteoporosis
- Chronic Sports Injuries
- Degenerative Joint & Disc Disease
- Chronic Sprains and Strains
- Cervical, Thoracic, and Lumbar Spine Strains
- Arthritic Joints
- Shoulder Pain, Hip Pain, and Knee Pain
- Ligament Laxity or Tears
- Tendon and Ligament Injuries
- Carpal Tunnel Syndrome
- Fine Lines and Wrinkles
- Traumatic Brain Injuries
- Anti-Aging Treatments
Regenerative Plasma
Concentrate (RPC) Procedure
RPC is an enhanced, ozonated plasma cocktail designed for advanced regenerative medicine. This powerful formulation combines concentrated platelet growth factors, stem cells, nutrients, and physician-selected peptides to support deep tissue repair, pain relief, and accelerated healing.
At our regenerative medical clinics in Aspen and Basalt, Colorado, your plasma is collected through a simple blood draw and transformed into a highly potent regenerative plasma concentrate. While the RPC is being prepared, you’ll receive a nutrient-rich IV to support whole-body recovery.
Once ready, the treatment includes a local anesthetic, targeted RPC injections for pain, sports injuries, and tissue damage, and a final therapeutic ozone shot to enhance cellular repair.
Each session lasts approximately 2–3 hours. Most patients experience a recovery period of 3–5 days, with optimal results appearing over 1–3 months.*
Results may vary; no guarantee of specific outcomes.
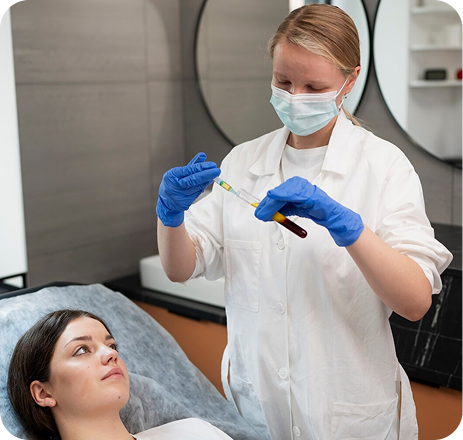
What Does Regenerative Plasma Concentrate (RPC) Cost?
The below cost includes the entire injection procedure (which may consist of several injections in one visit) as well as IV infusion.
Small Joint (toes, fingers, wrist, elbow, ankle): $5,500
Med Joint (knee, shoulder): $7,000
Back/Neck: $8,500
Extra Area: $3,000
Please note that these services are not billed to insurance. However, we can provide a superbill upon request to assist with potential out-of-network reimbursement.
Scientific References and Stem Cell Research
FAQs
Stem Cells FAQ
Basically a “blank” cell, an adult stem cell is capable of becoming another cell type in the body, such as a skin cell, a muscle cell, or a nerve cell. Adult stem cell therapy can be administered to help replace or even heal damaged tissues and cells, serving as a built-in repair system for the human body and replenishing other cells.
Recent stem cell research by the University of Texas Health Science Center at Houston (UTHealth) suggests that this cellular therapy can help reduce neuroinflammation, preserve brain tissue, and improve cognitive function following traumatic brain injury (TBI). Meanwhile, other regenerative medicine studies have indicated that adult neural stem cells have regenerative and reparative properties for the treatment of injuries or diseases in the central nervous system.
By definition, stem cells are undifferentiated biological cells that can differentiate into specialized cells and divide to produce more stem cells. There are three ways adult stem cells can be obtained: from bone marrow, adipose tissue, or blood.
Unlike embryonic stem cells, the use of adult stem cells in research and therapy is not considered to be controversial, as they are derived from adult tissue samples rather than discarded human embryos. By law, these adult stem cells have to be injected or infused into the patient within 24 hours after being obtained.
Adult stem cells have the ability to divide and generate all cell types of the organ from which they originate. They possess two properties: self-renewal and multi-potency, meaning they can go through numerous cycles of cell division while still maintaining their undifferentiated state. Stem cells hold the ability to generate into several distinct cell types, including neural cells found in the brain.
Regenerative stem cell therapy can stimulate tissue re-growth and greater blood flow to the affected areas.* The goal of treatment with stem cells is to replace damaged cells and to promote the growth of new blood vessels and tissues in order to help the target organ function at a greater capacity.*
*Results may vary; no guarantee of specific results
At Aspen Integrative Medicine, our peripheral blood-based stem cells (pulled from the patient’s own blood) are pluripotent and non-invasive. Pluripotent adult stem cells, those derived from the blood, have the ability to divide and generate all cell types and are considered “uncommitted”. These stem cells hold the ability to generate several distinct cell types, including neural cells found in the brain. Pluripotent stem cells behave like embryonic stem cells and give rise to all the cell types in the body. They endure a long lifespan and work best in combination with PRP.
Aspen Integrative Medicine only uses pluripotent stem cells in an effort to obtain the best results possible! Regenerative stem cell therapy can stimulate tissue re-growth and greater blood flow to the affected areas.* The goal of treatment with stem cells is to replace damaged cells and to promote the growth of new blood vessels and tissues in order to help the target organ function at a greater capacity.*
*Results may vary; no guarantee of specific results
Multipotent adult stem cells, those derived from bone or adipose tissue, have the ability to divide and generate all cell types of the organ from which they originate. They carry many growth factors, much like PRP, to help regenerate the same types of tissue they are taken from (fat and bone), unlike TBI Therapy’s peripheral blood stem cells, which are pluripotent and can turn into any type of tissue.
Amniotic fluid is what surrounds the baby. When the mother’s water breaks, this is the fluid that ends up on the floor. While amniotic fluid likely contains helpful growth factors and some “extracellular matrix,” it is not a stem cell product. See https://www.regenexx.com/amniotic-stem-cell-treatment/
Warnings: Be cautious about using cord blood or amniotic cells from another person due to widespread environmental contamination. Maternal exposure to some PFAS has been linked to altered thyroid hormone levels in maternal and umbilical cord blood. Chemicals designed to repel oil and water, known as PFAS (per- and polyfluoroalkyl substances), have been used widely in everything from food wrappers, cosmetics, and textiles, to firefighting foams. As a result, they have been detected in our food, water, and our bodies. Most forms of regenerative medicine are still in the early stages of development and adult stem cells and stem cells from birthing tissues have not yet been shown to be safe and effective for use in the treatment of any other diseases or conditions. The FDA issued a warning letter to Cord for Life, Inc., located in Altamonte Springs, Florida, for manufacturing unapproved umbilical cord blood products in violation of current good manufacturing practice (CGMP) requirements, including failing to validate processes to prevent bacterial contamination, raising potential significant safety concerns that put patients at risk. In addition, the FDA issued 20 letters to separate manufacturers and healthcare providers across the country who may be offering unapproved stem cell products, reiterating the FDA’s compliance and enforcement policy.
Most umbilical cells are not pluripotent but multipotent. There are currently several limitations to using Umbilical Cord stem cells. Although many different kinds of multipotent stem cells have been identified, Umbilical Cord stem cells that could give rise to all cell and tissue types have not yet been found. See: http://www.cordbloodsolutions.com/EmvsUm.aspx?AspxAutoDetectCookieSupport=1
“I don’t think the umbilical stems did anything, it was a couple of years ago.
I also heard a podcast and the doc said all the umbilical cells are dead.”
-patient report
Warnings:
Be cautious about using cord blood or amniotic cells from another person due to widespread environmental contamination. Maternal exposure to some PFAS has been linked to altered thyroid hormone levels in maternal and umbilical cord blood. Chemicals designed to repel oil and water, known as PFAS (per- and polyfluoroalkyl substances), have been used widely in everything from food wrappers, cosmetics, and textiles, to firefighting foams. As a result, they have been detected in our food, water, and our bodies.
Most forms of regenerative medicine are still in the early stages of development and adult stem cells and stem cells from birthing tissues have not yet been shown to be safe and effective for use in the treatment of any other diseases or conditions.
The FDA issued a warning letter to Cord for Life, Inc., located in Altamonte Springs, Florida, for manufacturing unapproved umbilical cord blood products in violation of current good manufacturing practice (CGMP) requirements, including failing to validate processes to prevent bacterial contamination, raising potential significant safety concerns that put patients at risk. In addition, the FDA issued 20 letters to separate manufacturers and health care providers across the country who may be offering unapproved stem cell products, reiterating the FDA’s compliance and enforcement policy.
Contact Aspen
Integrative Medicine

BASALT:
227 Midland Ave, Suite 18B, Basalt, CO 81621
(In the courtyard between Basalt Firearms and Karen White)
Hours: Tuesday & Thursday: 12pm-5pm
Appointment required
ASPEN:
230 S. Mill Street, Aspen, CO 81611
(Above Royal Street Fine Art and Across from Louis Vuitton)
Hours: Monday, Wednesday, Friday: 12pm-5pm
Appointment required
